According to Alfields LLC., a company headquartered in Abu Dhabi, the company's researchers have discovered a new carbon allotrope, protomene, which may be more suitable for optoelectronic components than gallium nitride (GaN). It is also suitable for more semiconductor component applications than carbon nanotubes (CNTs) and graphene. Research on this topic was published in the most recent issue of the scientific journal Carbon, where researchers explore the structure of this new carbon allotrope and believe it is likely to develop into a material for significant advances in the electronics industry.
The international expert research team dedicated to this research is led by the brothers Mohamed Al Fahim and Rashid al Fahim of Alfields LLC. The plan is also part of the innovation and future technology required by the United Arab Emirates government in response to the launch of the Fourth Industrial Revolution policy in September 2017.
Larry Burchfield, a US nuclear chemist and chief scientist at Alfields, said: "Protomene carbon allotropes and their important properties have been the wish list of forward-thinking innovators and manufacturers in recent decades, and now we will be truly Realizing this material." Burchfield said: "We have now jumped away from the 'dream' stage, and ultimately brought a very favorable influence in the fields of semiconductor, optoelectronics, coatings and energy conservation." Alfields said this may be the first new carbon allotrope type since Nobel Prize winners Robert F. Curl Jr., Sir Harold W. Krotoand and Richard E. Smalley discovered fullerenes. It is also the most significant progress since the Nobel Prize winners Andre Geim and Konstantin Novoselov discovered graphene in 2010.
The researchers further collaborated with the Khalifa University of Science and Technology in Abu Dhabi to develop the next phase of the actual manufacturing of protomene. Protomene has proven to be a promising new direct-gap semiconductor. Its band gap is very close to GaN - at room temperature, the energy gap of GaN is about 3.4 eV. Therefore, protomene has semiconductor properties similar to those of GaN, enabling it to be applied to high power and/or high frequency electronic components with high breakdown voltage.
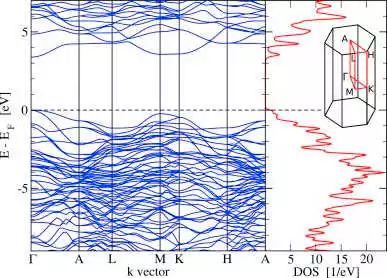
Protomene electronic state of the energy zone around the top of the valence band (dashed part) (Source: Carbon)
However, since GaN is a two-position compound, it is difficult to control the composition during crystal growth, and protomene is a carbon allotrope of a single element, and the degree of mastery of defects may be better than that of GaN. Since the gap amplitude is near the blue end of the visible spectrum, protomene is expected to find new applications in optoelectronic components, such as blue or ultraviolet (UV) light that produces LEDs, or as UV filters for optics.
Furthermore, from the viewpoint of energy gap, protomene may be more suitable for many semiconductor components than carbon nanotubes and graphene. In fact, whether it is metal or semiconductor, one of the obstacles in the current manufacture of carbon nanotubes is to control it. Conversely, Protomene is expected to be a semiconductor that changes with temperature.
Explore new allotropes
The thermal expansion of protomene is likely to occur in the bonding between the plates. When the temperature rises, the structural phase transition may occur due to the structural change from the 48-atom unit structure of the low-temperature semiconductor to the 24-atom unit structure of the high-temperature metal. As the phase transition occurs, the energy gap will converge rapidly, even faster than the decay and thermal expansion in diamonds and silicon.
Therefore, this phase change will provide a sensitive temperature control optical filter. Eventually, it will be converted into high-temperature dimer-free metal of protomene, and it also has the potential to realize applications such as temperature-controlled photoelectric switches. The pursuit of new carbon isotopes has become an increasingly active area of ​​research for decades. Carbon allotropes have a variety of structural and electronic properties that contribute to a wide range of research interests.
Carbon typically has three highly competitive blends of different orbital domains—sp, sp2, and sp3. This allows the carbon atoms to be combined with one another in a number of different ways. The configuration of sp3 produces three-dimensional (3D) networks with insulating properties and high stiffness, such as cubes and hexagonal diamonds. In contrast, sp (linear) and sp2 (planar) blends enable flexible structures such as carbine and graphene, which typically have small inter-band energy gaps or even metallic properties. Intermediate blends are also common, such as fullerenes and nanotubes. Protomene is a new stable carbon structure based on the combination of sp2 and sp3, in which 6 of the 24 atoms can adopt a completely planar sp2 geometry and thus can be removed from the plane, and in the next vertical stack of crystal lattices The paired atoms form a relatively weak bond. This additional bond formation will reduce the total energy by about 1 eV per bond, causing a significant change in electronic properties.
Led Beam Moving Head,Beam Moving Head Light,Mini Led Moving Head Light,Moving Beam Light
Guangzhou Cheng Wen Photoelectric Technology Co., Ltd. , https://www.cwledwall.com